ISO 17025:2017 Laboratory Quality Manual & Procedures
Save time and money with our fully customizable Laboratory Quality Manual and Procedures Package. Our processes are well-organized and carefully designed to work together to lead your organization to continuous improvement.
- Documents in Microsoft Word or Excel for easy customization.
- Together, the docs include the content required to address each requirement of the ISO/IEC 17025:2017 Standard
- Very clear instructions in obvious Blue Text to show you where to customize your Manual and Procedures. Treat the text in blue as “revisions” or information that is specific to your company.
- Docs are all numbered for an effective control of documented information and are integrated to work together in a seamless system.
- Related documents are referenced for effective record keeping.
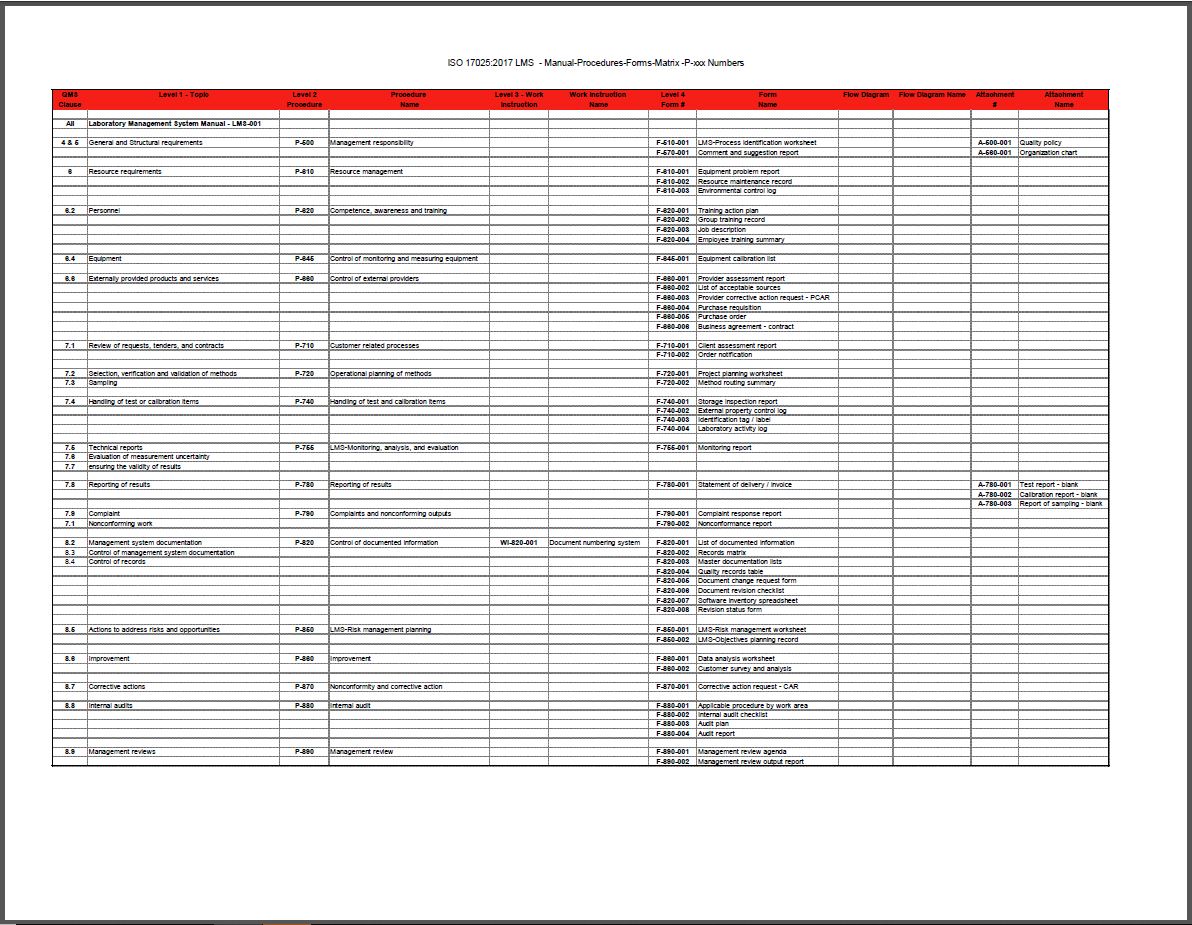
- Intuitive architecture for easy Document Control. We’ve numbered QMS documents to correspond with the sections of the 17025 Standard. Accreditors LOVE this feature!
- This is a downloadable product.
As you implement your ISO 17025 system you will also need checklists and training. You can save time and money by purchasing our Certification Packages!
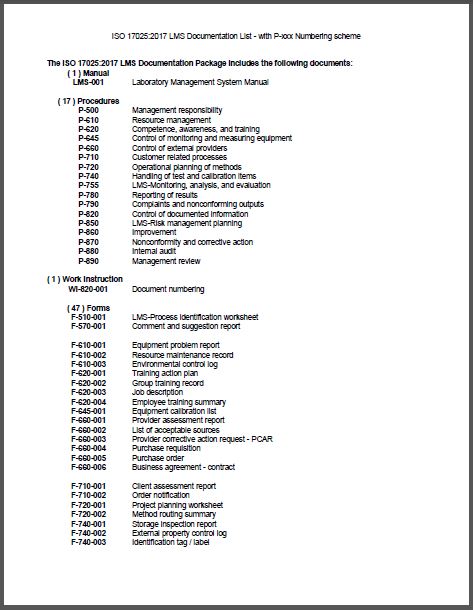
The documentation package for the management system will contain:
- (1) condensed Manual to introduce the documented information required for ISO 17025:2017.
- (17) procedures outlining how you will control each requirement of the standard
- (1) work instruction outlining document numbering system
- (47) forms to record the data required by the procedure
- (5) attachments
- (3) blank, formatted templates to create your own additional documents
- Technical Support
Click below for a sample of the Quality Manual, Procedures, and Forms:
Compare Products
Which version is right for me?
If you plan to reconfigure your existing quality manual and procedures to meet the ISO 17025:2017 standard, use Upgrade Instructions to create everything on your own. They will tell you where to make the changes but will NOT provide templates or new verbiage on the new requirements.
ISO 17025:2017 LQMS is for those just creating their first ISO Management System, and provides necessary documentation to meet the requirements of ISO 17025:2017. See the informational box above for samples, contents, and more information.
If you are transitioning from ISO 17025:2005 to ISO 17025:2017, the LQMS Upgrade includes the QMS + instructions. This provides you a map of where to copy some text from your existing QMS, and place it in a new QMS structure which follows Annex L.
| Price Each | 17025:2017 QMS |
17025:2005 to 17025:2017 QMS |
|
|---|---|---|---|
| Your Price | |||
| ISO 17025:2017 Laboratory Quality Manual (not sold separately) | $149 | ||
| ISO 17025:2017 Procedures | $400 | 17 | 17 |
| ISO 17025:2017 Forms and Attachments | $109 | 52 | 52 |
| ISO 17025:2005 to 2017 LQMS Upgrade Instructions | $299 | ||
| Support | FREE | ||
| Your Price |